It's a popular pick when you're feeling under the weather, but one U.S. agency is warning it could pose a possible poisoning risk.
More Than 14,000 Boxes of Cold and Flu Medicine Have Just Been Recalled

In total, health experts estimate there have been 45 million flu cases this season. And though the peak of flu season seems to be behind us, recent data shows this year has seen a “high severity season overall and for all ages groups,” the first with this classification in seven years.
If you were unlucky enough to come down with the flu, you know just how helpful taking an over-the-counter medicine can be for some relief. Unfortunately, anyone experiencing flu symptoms now should be aware of a medicine that’s been recalled.
On Thursday morning, the U.S. Product Safety Commission (CPSC) released important information about one particular cold and flu medicine—for a reason we’ve seen in several recent recalls.
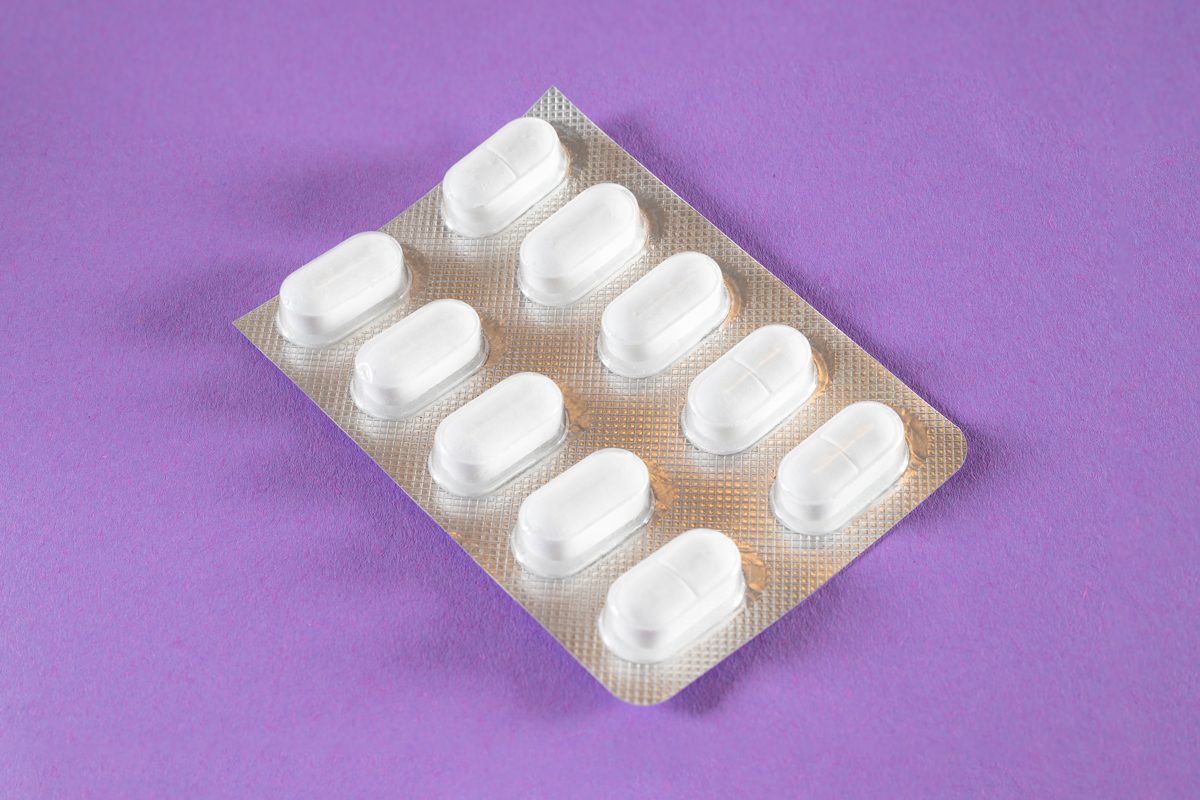
According to the CPSC’s report, approximately 14,250 units of Safetussin Max Strength Multi-Symptom Cough, Cold and Flu have been recalled nationwide because one of the medicine’s ingredients, acetaminophen, requires child-resistant packaging due to the Poison Prevention Packaging Act. However, the recall announcement notes, “The packaging of the products is not child-resistant as a tablet can be pushed through the foil, posing a risk of poisoning if the contents are swallowed by young children.”
Consumers can identify the affected product by the following details:
- The medicine was sold in blue, orange, and red cardboard boxes containing 24-count caplet blister packs.
- Drug facts are printed on the back of the box.
- The packaging has the following text: “Safetussin,” “Multi-Symptom,” “Cough, Cold & Flu,” and “Safe for adults with High Blood Pressure, Diabetes.”
- The medicine was distributed by Kramer Laboratories of Bridgewater, New Jersey.
The CPSC says the recalled medicine was sold at HEB, Harris-Teeter, and other regional grocery stores, as well as independently owned pharmacies, nationwide from July 2024 through March 2025 for about $11.
Acetaminophen, used for relieving both fevers and pain, is “an active ingredient in hundreds of over-the-counter (OTC) and prescription medicines,” according to the U.S. Food and Drug Administration (FDA). But taking too much acetaminophen can cause serious liver damage, which is why households with little ones should especially note the recall. In fact, the CPSC recommends consumers “immediately secure the product out of the sight and reach of children.”
Concerned parents should also be aware that Benadryl and iron supplements have been recalled in the past few weeks for similar reasons related to a lack of child-resistant packaging.
Safetussin Max Strength Multi-Symptom Cough, Cold and Flu customers are advised to contact Kramer Laboratories—either by phone at 800-824-4894 or email at [email protected]—for information on how to return or dispose of the product for a full refund. Though the medicine in the packaging is not being recalled, the CPSC says both the packaging and medication should be disposed of.
For daily wellness updates, subscribe to The Healthy by Reader’s Digest newsletter and follow The Healthy on Facebook and Instagram. Keep reading:

















